|
 |
 |
 |
 |
 |

Neuroplastic Pain
Neuroplastic Pain: refers to pain cause because of changes within the nervous system. These structural and functional changes can occur at every level of the
nervous system.
Traditionally, pain has been regarded as being either nociceptive
(arising in response to tissue injury) or neuropathic (arising in
response to nerve injury). Although this distinction has had some
therapeutic utility, it has served to maintain the Cartesian concept of
a fixed immutable pain system that faithfully transmits information
from a site of injury to pain centres within the brain. Although this
is largely true after acute injury, it is clear from epidemiological
studies that in the presence of persistent disease a range of
additional factors, often unrelated to the musculoskeletal system,
serve to modify activity within pain (nociceptive) pathways.
Implicit in recent classification schemes is the notion that acute
and chronic pain states are different and that functional changes
within the nociceptive system are important in determining the signs
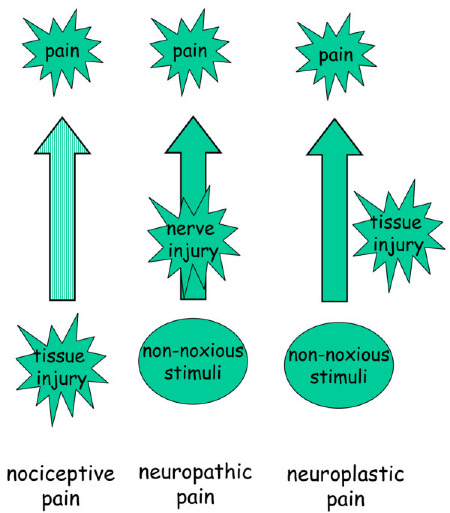
and symptoms experienced by individuals with somatic disease [2]. Currently, four different pain states are recognized (Figure 1).
The first of these, nociceptive pain, refers to those transient
symptoms and signs that arise in response to acute injury and reflects
the activation of specialized pain receptors (nociceptors) and
corresponding activity in more central pathways. Under these
conditions, symptoms broadly reflect the initiating stimulus or injury;
treatment at a peripheral level is likely to be successful. |